Does Molnupinavir reduce the risk of long COVID?
An open label study claims it does; Good critical appraisal says that paper proves nothing
Recently some researchers* have claimed that the PANORAMIC randomized trial is the first to show an anti-viral can prevent long COVID. Shall we assess the trial and the claim?
This is the paper
It is a randomized trial of molnupinivir vs. usual care. NOTE: It is not a placebo controlled trial. The control group gets nothing.
And, If you get the new drug, the intervention is delivered in style.
“Participants in the molnupiravir group were… urgently couriered a participant pack containing molnupiravir”
UberEATS for Molnupiravir.
Notably that was negative.
“Hospitalisations or deaths were recorded in 105 (1%) of 12 529 participants in the molnupiravir plus usual care group versus 98 (1%) of 12 525 (1%) in the usual care group (adjusted odds ratio 1·06 [95% BCI 0·81–1·41]; probability of superiority 0·33)”
That’s disappointing. Note the low event rate in the study. COVID was already on its way to being a cold at the time of the study, and since then it has even lower event rates. This is also why PANORAMIC’s study of Paxlovid is taking so long. If it were hugely positive, or the event rate brisk, it would have resulted by now.
Enter the recent study. It is a secondary analysis looking at the symptoms of long covid. Indeed, it looks like the arm that got the drug has fewer long covid symptoms.
The raw numbers however are small, just a couple percentage points.
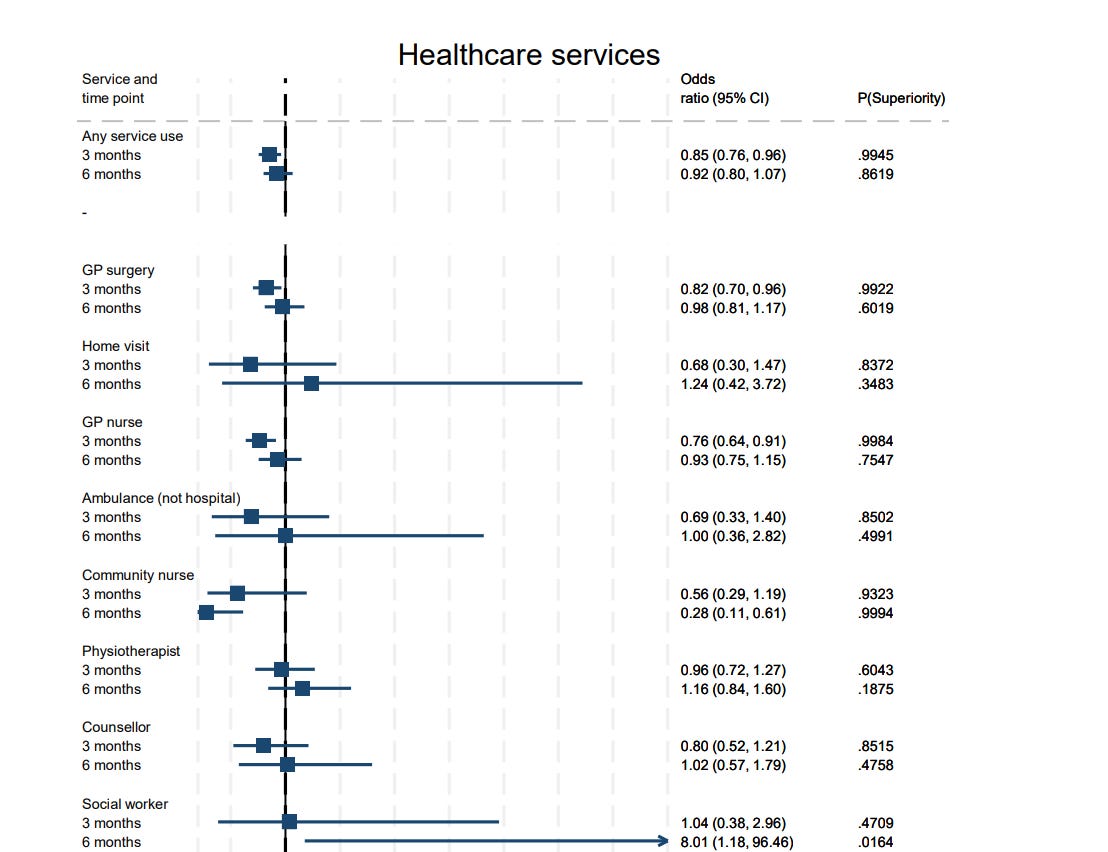
And there are small differences in health care usage
But the big problem with this study is this is OPEN LABEL. Patients either got someone to deliver a new drug to them via a urgent courier service or usual care. I am sure the group that got the pill has less long COVID but is that because the chemical in the pill helps, or because of the placebo effect? They feel taken care of, getting rapidly delivery of a new pill.
In general, unblinded studies are good for very hard, bias resistant endpoints. COURAGE was unblinded RCT of stents or pills and the primary endpoint of death or heart attack is bias resistant. But the secondary endpoints of symptoms require BLINDING. Hence the need for ORBITA— a blinded study that showed stents didn’t improve modified bruce protocol treadmill times when given on top of meds (as they should be).
Subjective symptoms— how you feel— is much more prone to the placebo effect than objective markers.
In fact, this bias is so obvious, the investigators should not have built in subjective secondary endpoints without placebo control.
If you read a study where people *feel better* make sure the control arm gets a placebo, or active placebo (it also has a metalic taste, for e.g.), or a sham proceedure, otherwise the results are unreliable, as they can’t separate placebo effect from real effect.
What if it is all just a placebo effect? It is still worth it, some may argue. In this case, however, why put molnupinivir in the tablet. Just make it a package of smarties and say it helps long covid— there is less risk from a pure placebo than a chemical that acts solely as placebo.
So when *some researchers say this. They are wrong.
Naturally, Eric Topol uncritically reports this study
Be careful if you follow Eric Topol on twitter; He often promotes papers without proper critical appraisal.
This is a reader supported publication. Paid subscribers get to leave comments and questions and your support keeps this going.







Topol is an NPC.
Wel what could go wrong with molnupinavir. Its mode of action is to induce a large number of mutations in the virus thereby preventing replication. That's all very well, but it is also going to cause mutations in genomic DNA so there is a real risk of causing cancer long-term. That would be a huge disaster for a viral illness that currently is nothing more than a cold and where the existence of so-called long-covid, as opposed to post-viral syndrome associated with many upper respiratory tract viruses, is dubious at best.