FDA approves Pfizer drugs with inadequate safety and efficacy data, and then regulators work for Pfizer
This is corruption
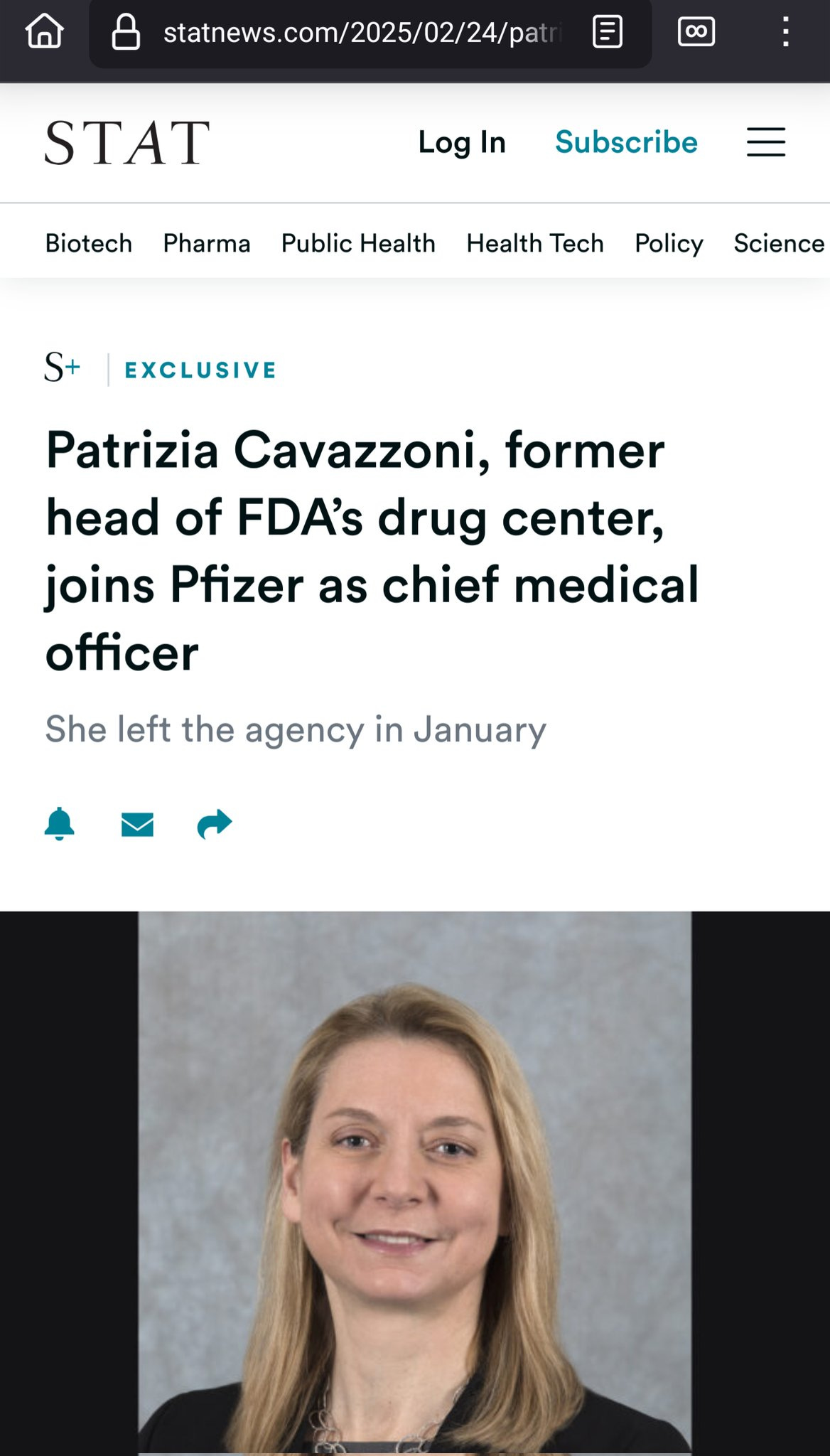
Breaking news: FDA CDER head has left FDA and joined Pfizer
That is right. The CDER director, overseeing the approval of numerous Pfizer products has left the FDA to work for Pfizer.
Here is how American drug regulation works:
FDA regulators set arbitrary benchmarks for novel products. For instance, does your new sickle cell drug increase hemoglobin by 1 point?
It is important to set arbitrary benchmarks. If you had no benchmarks then anyone could enter the market, and prices would fall. You need some hurdles to ensure only large (enough) firms can make it to market, and there are limited new drugs, so that prices can be kept high. We need some hurdles.
But they can’t be too high. If, instead, you used endpoints like, “do sickle cell kids have less pain and do they live longer?” well then Pfizer drugs might not succeed. So the hurdles can’t actual be net or composite measures of patient benefit.
Next, companies get their products approved, and the FDA ‘works with them’ to make sure that happens smoothly. The FDA can help companies by deciding what information is confidential and private.
Sometimes the FDA gets the opinion of outside experts, but it makes sure to stack those panels with people being paid by Pharma. The vote tends to go in Pharma’s direction, but if it doesn’t FDA sometimes overrides the vote and goes with pharma anyway (this is not a joke— we have empirical data on this)
Then, after a few years on the market, your Pfizer drug product might be found to have safety problems. Maybe it even killed a few kids with sickle cell disease. Who would even notice?
Now, you really have to help Pfizer, by writing a vague press release— failing to distinguish between death and vaso-occusive disease, so that it is harder to write damning media stories about this product.
Then, after a few years, when your LinkedIn has enough connects, you leave the FDA and go to work at Pharma. Ideally, you work for the same company whose products you regulated favorable. And they pay you handsomely.
In a normal world, this is corruption, but in Medicine, it is just a footnote.
This is the core rot in American regulation. The revolving door politics. I find this behavior abhorrent, and it should be criminal. Mr. Kennedy has vowed to stop this, and I welcome that. Until it happens, American medicine labors under a curse: we cannot trust our drug regulators to act in the public’s interest. Instead, they too often are acting in their own.




When is Makary up to be confirmed? We need him ASAP
A terrific ejaculate of information from Vinay that clearly supports at every turn of the prose his prior feelings about a Fundamentally Dirty Association (FDA). Vinay has now developed what is termed "having a red ass" down in rural west Texas. That diagnosis traditionally can be only be made after a long period of "having a burr under the saddle blanket".