Open concerns with recent RCTs for Maternal RSV vaccination
Increased preterm birth and lack of effect on all-cause lower respiratory tract infections are serious limitations to the evidence base and should warrant larger RCTs
I’ve been concerned for some time about the maternal RSV vaccine(s). Last year, Dave Allely (Med student Mt. Sinai) and I wrote to the authors of the MATISSE study in the New England Journal of Medicine and asked them to clarify the impact on all lower respiratory tract illnesses of any cause, and other questions, including preterm birth information. Specifically we write:
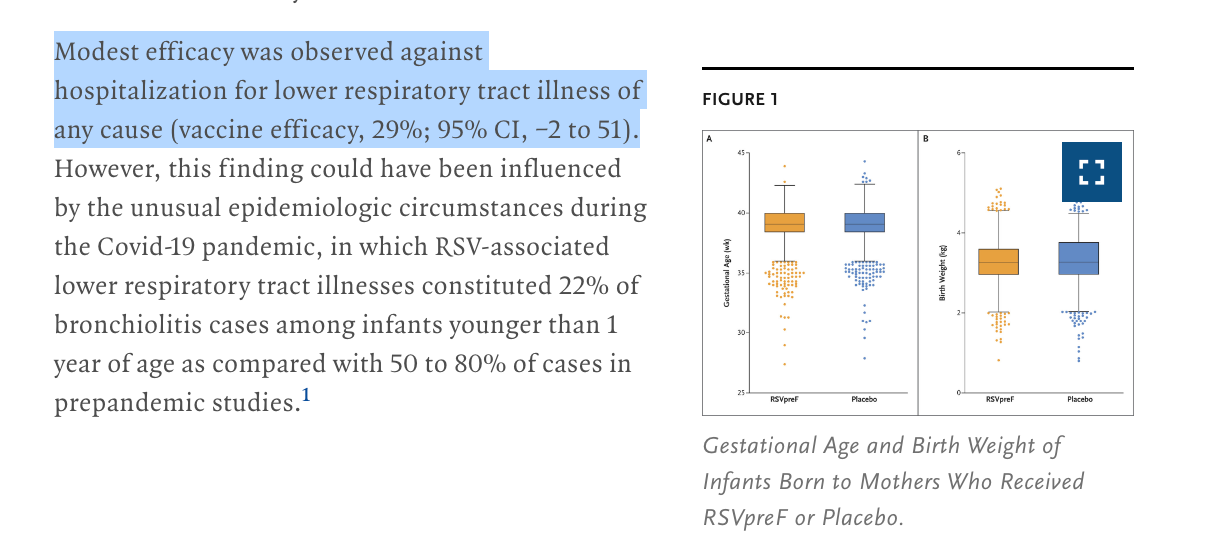
In response, the authors noted that “incidence of preterm birth (birth at <37 weeks’ gestation)” was higher if you got the RSV vaccine than if you did not, thought this was not statistically significant. Specifically, a “5.7% (95% confidence interval [CI], 4.9 to 6.5) in the vaccine group and 4.7% (95% CI, 4.1 to 5.5) in the placebo group”
Moreover, they noted that there was no statistically significant reduction in hospitalization for lower respiratory tract illness of any cause. Yet, here they used the word “modest efficacy”. Note: the CI crosses 0.
It struck me that this was a double standard. If a non-significant difference can be called “modest efficacy,” a 1% numerical increase in preterm birth (also not significant) should be a modest harm.
Now a new paper is out in NEJM that suggests that low-birth weight might be a class effect. This trial was stopped for a 2% increase in pre-term birth.
This trial also found a non-significant increase in neonatal death:
“neonatal death occurred in 0.4% (13 of 3494) and 0.2% (3 of 1739), respectively (relative risk, 2.16; 95% CI, 0.62 to 7.56; P=0.23), an imbalance probably attributable to the greater percentage of preterm births in the vaccine group”
This study— by GSK— does not report all cause lower respiratory tract hospitalization.
Putting it together:
We have 2 studies, and both show numerically higher rates of pre-term birth. One is significant. The other is not. One study shows no reduction in all lower respiratory tract hospitalization and the other does not report it. One study shows increased neonatal death, though this is not significant.
Looking at these results it is impossible to know if the vaccine is a net positive or net negative. Focusing on only lower respiratory tract hospitalization from RSV is not a fair way to judge efficacy. Those gains should result in lower rates of hospitalization for any respiratory virus, and ideally overall better health. Those gains have not been demonstrated.
Meanwhile, more preterm birth appears to be a class effect, and there is a concerning trend with neonatal death in the GSK study.
Researchers sometimes call non-significant results “modest efficacy” but don’t call non-significant harm— “modest risks”.
Ultimately, I have no confidence this vaccine is a net positive— larger studies are needed— and they need the power to find the effect size if one breast feeds vs. if one does not.
Again, the FDA is pushing products with uncertain benefit to harm balance.




In other words this is a product being given to healthy young people with no definite data supporting its benefit and data that are suspicious for a net harm … is that a fair assessment?
Vinay, please give us your observations on the ADULT RSV vaccines too. I have held off on recommending until there was more safety data regarding new onset a-fib and guillain barre syndrome. I do not trust info from CDC anymore.