Red Dye #3 is out, and boosters may get RCTs: Why is the evidence to remove a chemical from food LESS THAN the evidence to recommend a medical interventions?
Things we don't teach well in medical school
Today the US FDA announced that Red Dye #3 will be banned in the US.
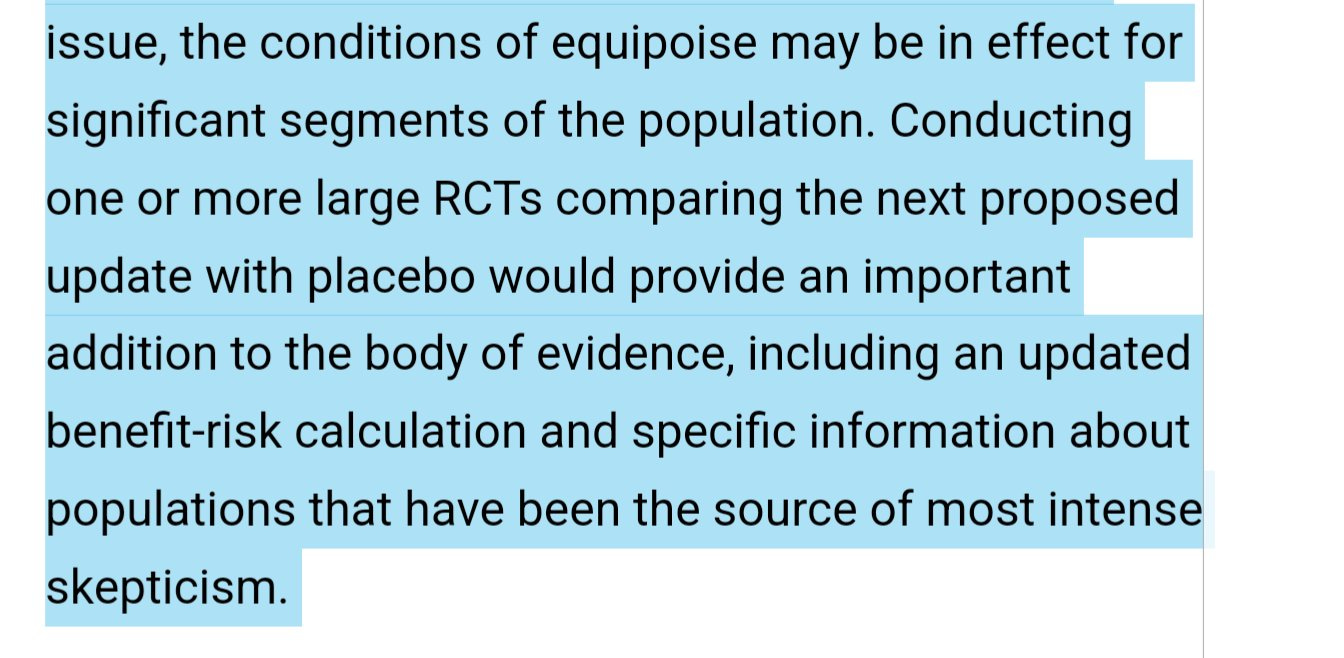
A day ago, the FDA commissioner Bob Califf— who never asked for a randomized trial powered for clinical endpoints of the booster— suggested we should get one. (Please note, I have said this for years).
What is going on: Since when did the Biden administration become MAHA?
And….
Why do we need RCTs of boosters, but we don’t need an RCT to remove red #3?
I worry that most medical doctors don’t understand that the evidence to remove a potentially harmful, unnecessary chemical substance from food SHOULD BE different than the evidence to recommend a costly, injectable, novel medical product. Let me briefly explain.
In medicine, nearly all of our new interventions cost money, have side effects, and represent opportunity cost. An annual booster makes a sizable percentage of recipients sick for a day or so, rarely it can cause myocarditis or other major harm. It also costs billions of dollars. It may have long term effects we don’t know because the medical profession is discouraged from investigating side effects, and existing US surveillance systems are not good.
Given these factors, and that boosting well adults and kids means, by definition, encouraging or coercing the healthy, there is a scientific and moral obligation to run large, well done randomized studies to know if an intervention works prior to deployment (or at least concurrent with).
Similarly, before you deploy routine palliative care into EDs for adults with life limiting illness— spending all that money that could be spent elsewhere— a cluster RCTs might be ideal to know if it improves outcomes around death or dying. Spoiler alert: it didn’t in a recent JAMA paper.
Now consider the case of Red Dye #3. Is there randomized evidence that the dye causes cancer? No, there is no such human trial to my knowledge. Why is the burden of evidence not the same: don’t remove it, unless you know for sure it causes cancer? Because, the standards around what non-beneficial substances should be added to food and cosmetics are different than the standards around what beneficial substances we should encourage you to ingest.
In the case of medical interventions, the claim is: doing this benefits you. Thus, the burden is to show that benefit. In the claim of food additives the claim is: this substance has no positive effect. No one says Red Dye #3 makes you taller. It might make kids more excited to eat your sugary snacks (probably the real motivation to keep it). The burden then is to show that it is not deleterious. Any suspicion it might be deleterious may be deemed unacceptable, because at the outset we stipulated: it has no benefit. To prove something is truly no worse than not eating it, you would have to do a MEGA— multi million person RCT powered for non-inferiority. The burden of proof cannot be on the state to run such a study because only an infinite large study can exclude all risk.
Think of it another way. A chemist can synthesize 1000 new molecules a month. If he calls these drugs, do we let him sell them all? No, we ask for him to prove his snake oil claims. What if he claims the same chemicals are food additives? Do we suddenly change our tune and allow all of them into food until someone proves they are harmful? No. When it comes to injecting substances that have no putative claim of benefit, even weak claims of harm are sufficient to remove them, and moreover, a high bar should exist to introduce them in the first place. There are more ways to make someone sick than healthy.
When people talk about the precautionary principle, this is what they mean. It means both: lets be cautious about injecting Tommy, an 8 year old, with a covid booster that only has been given to mice given that Tommy had COVID last month, is 8 (aka at low risk of bad outcomes), and given that we don’t have long term safety data (or super solid short term data) for this novel mRNA construct.
It also means: lets be cautious about cooking Tommy’s pancakes in this industrial grade oil product that Dow Chemical found on the bottom of the distillery column because we could use butter, which has existed for thousands of years, and has never had a problem.
(This is also my problem with the seed oil debate. We used to make fries in tallow. They tasted good. Then some moron said vegetable oil is healthier. That was a lie. No evidence supports that. So we should never have switched. But now the same moron is back saying to prove vegetable oil is harmful. Listen asshole, we moved away from tallow because you were lying, and now we caught you, so we don’t have to prove it is harmful. The fries taste like shit now. They are floppy and lack proper crunch. They aren’t fluffy enough in the center. Anthony Bourdain never switched because he knows what a fry should taste like and he is an idiot like you. Also the oil is aerosolizing and sticking up the ceiling tiles. So, we should switch back without having to prove anything to you. Go back to working on your alcohol meta-analysis that shows even looking at wine is harmful. Bye now.*)
The burden of RCTs exist for those who offer, sell or deploy substances THEY CLAIM make you better off, but not those who think we should add novel chemicals to our bodies that are done for CONVENIENCE or COLOR or PRESERVATION— these individuals face a much different bar. If all animal and preclinical data is assuring, we might allow the addition. We will monitor carefully, and even a weak suggestion of harm will be grounds to dismiss because we have alternatives, this is not strictly necessary, and no one thinks it is good for you anyway.
Robert Califf failed during this term at FDA. He authorized booster after booster, cancer drug after cancer drug, without good RCT data. Without good RCT data, he lied and said Paxlovid lowers the rate of long covid. (He said anti-virals). He also did nothing to remove the thousands of chemicals the US permits in the food that Europe doesn’t. He didn’t even think about it. I dunno what he was doing: perhaps dreaming of the companies he would work for after he is done at the FDA. Then he hears that RFK Jr and Marty Makary are coming for his agency, and he does both these things as he is leaving so that he can try to save face. To me, that exemplifies being a spineless regulator.
The burden of proof to recommend a medical product is higher than remove a potential toxic food additive from your meals. This is perfectly logical and consistent, and folks who think otherwise need a better education.
And good fries are good in tallow.
If you support me, please subscribe. We know Bob Califf won’t be
*since my audience cannot always tell, this is also a joke.



Meanwhile, latest JAMA has
- a study showing vaccines double apneic events in preemies
- a study showing a link between fluoride and IQ drop
Do either of these get published if Kamala wins the election?
I'm not $ure. I a$$ume there'$ a logical rea$on, but I'm ju$t a $illy civilian out$ide of the medical community.